Plant Nutrients in the Soil: Plants receive their nutrients mainly from soil and this soil act as a living source for plants to fulfil the nutrients necessity, needed by plants, for their normal growth, development and reproduction. The nutrient elements must be directly involved in the metabolism of the plant. In the absence of the element the plants do not complete their life cycle or set the seeds.
Table of Content
Plant Nutrient in the Soil
There are 17 Essential Elements that act as essential plant nutrients for growth, are: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), Phosphorus (P), Potassium (K), Sulfur (S), Calcium (Ca), Magnesium (Mg), Boron (B), Chlorine (Cl), Copper (Cu), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), and Zinc (Zn).
The three main nutrients are nitrogen (N), phosphorus (P) and potassium (K). Together they said to be or known as NPK. The three macro-nutrients that plants can obtained from water, air, or both— Carbon (C), Hydrogen (H) and Oxygen (O) while micro nutrient for plant include iron, manganese, copper, molybdenum, zinc, boron, chlorine and nickel.

[Note: In addition to the 17 essential elements named above, there are some beneficial elements such as sodium, silicon, cobalt and selenium. They are required by higher plants.]
Micro and Macro Nutrients in Plants
The above 17 essential plant nutrients are further divided into two broad categories based on their quantitative requirements as micro and macro nutrients in plants.
(i) Macronutrients, and
(ii) Micronutrients
Macronutrients are generally present in plant tissues in large amounts (in excess of 10 mmole Kg –1 of dry matter). The macronutrients include carbon, hydrogen, oxygen, nitrogen, phosphorous, sulphur, potassium, calcium and magnesium. Of these, carbon, hydrogen and oxygen are mainly obtained from CO2 and H2O, while the others are absorbed from the soil as mineral nutrition.
Micronutrients or trace elements, are needed in very small amounts (less than 10 mmole Kg –1 of dry matter). These include iron, manganese, copper, molybdenum, zinc, boron, chlorine and nickel.
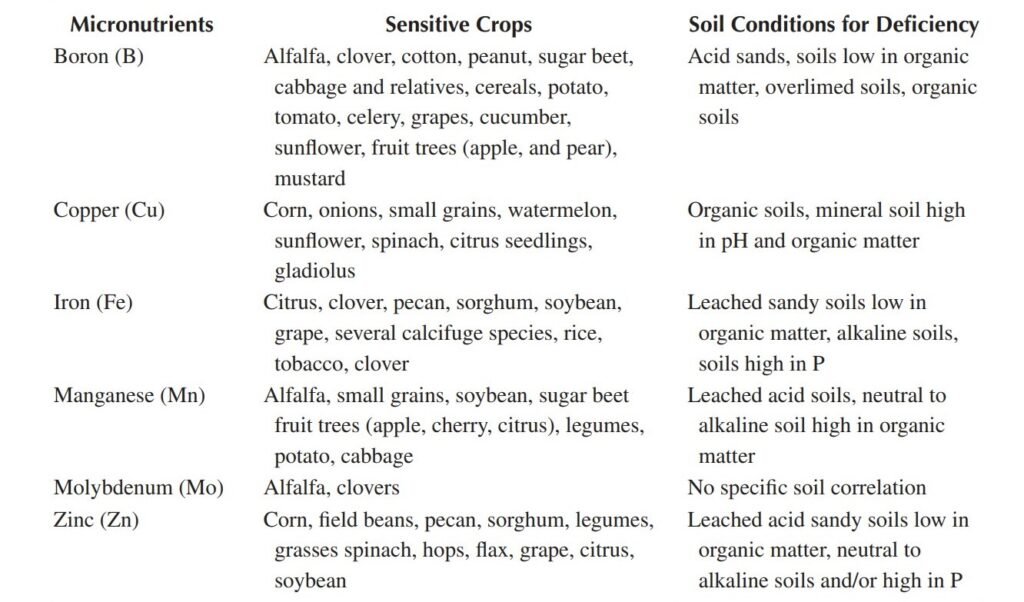
Some crop plants, that are uniquely sensitive to either deficiency or excess of micro and macro nutrients in plants.
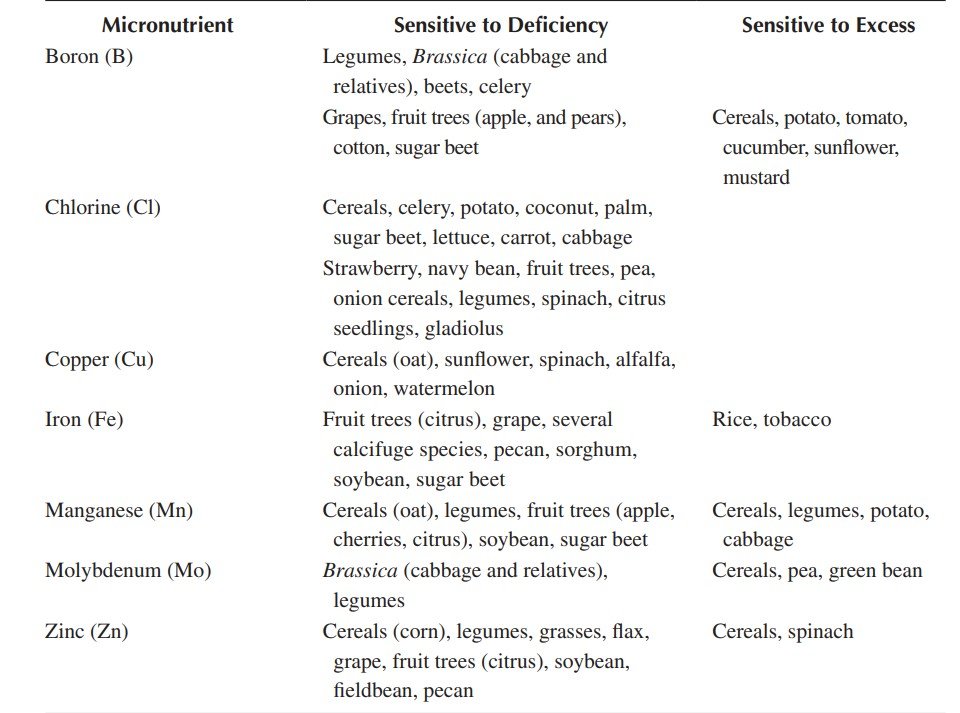
Essential Plant Nutrients and Nutrient Deficiency in Plants
17 Essential plant nutrients and their functions, included with plant nutrient deficiency symptoms in plants & identification.
Major Elements
Nitrogen (N)
Established Year: 1802 (de Saussure) and 1851–1855 (Boussingault)
Researcher(s): de Saussure and Boussingault
Existence:
Nitrogen is a base element, In plant growth it is found in all plant cells, in plant proteins and hormones, and in chlorophyll too.
Source: Atmospheric nitrogen is a source of soil nitrogen. Plants take up N from the soil as NH4 + (ammonium) or NO3 – (nitrate)
Some plants such as Legumes, fix atmospheric nitrogen in their roots;
Fertilizer: Fertilizer industries use atmospheric nitrogen from the air to make Ammonium sulfate, Ammonium nitrate and Urea. When applied to soil, nitrogen is converted to mineral form, nitrate, so that plants can take it up.
Organic matter: Soils high in organic matter such as chocolate soils are generally higher in nitrogen than podzolic soils.
Suggestion: Due to Soil acidification Nitrate is easily leached out of soil by heavy rain. You need to apply nitrogen in small amounts often so that plants use all of it, or in organic form such as composted manure, so that leaching is reduced.
Function of Nitrogen (N):
i) It makes plant dark green & succulent
ii) It promotes vegetative growth.
Phosphorus (P)
Established Year: 1839 (Liebig) and 1861 (Ville)
Researcher(s): Liebig and Ville
Existence:
Stimulates growth: Phosphorus is a essential plant nutrient that helps, transfer energy from sunlight to plants, stimulates early root and plant growth, and hastens maturity.
Source: The most common phosphorus source is superphosphate, made from rock phosphate and sulfuric acid. All manures contain phosphorus; manure from grain-fed animals is a particularly rich source.
Function of Phosphorus (P):
i) It stimulates root development, increases the number of tillers, gives strength to straw and prevents lodging.
ii) It hastens ripening of plants and counteracts the effects of excessive nitrogen.
iii) It improves the quality and yield of grain.
It increases disease resistance, enhances the activity of rhizobia and increases the formation of root nodules in legumes.
Potassium (K)
Established Year: 1866
Researcher(s): Bimer and Lacanus
Existence:
Source: Muriate of potash and sulfate of potash are the most common sources of potassium.
Resistance: Potassium is a essential plant nutrient that increases vigour and disease resistance of plants, helps to form and move Starch, Sugars and Oils in plants, and can improve fruit quality and make rich in quantity.
Function of Potassium (K):
i) Vigour and disease resistance to plants.
ii) It increases efficiency of the leaf in manufacturing sugars and starch.
iii) It helps to produce stiff straw in cereals and reduces lodging
Calcium (Ca)
Established Year: 1862
Researcher(s): Strohmann
Existence:
Stimulates growth: Calcium is essential for root health and is a essential plant nutrient, growth of new roots and root hairs, and the development of leaves.
Source: Lime, gypsum, dolomite and superphosphate (a mixture of calcium phosphate and calcium sulfate) all supply calcium. Lime is the cheapest and most suitable option for different prospects; dolomite is useful for magnesium and calcium deficiencies, but if used over a long period will unbalance the calcium/magnesium ratio. Superphosphate is useful where calcium and phosphorus are needed.
Function of Calcium (Ca):
i) Increases stiffness of straw and promotes early root development and growth.
ii) It encourages seed production
Magnesium (Mg)
Established Year: 1875
Researcher(s): Boehm
Existence:
Magnesium is a base element of chlorophyll, the green colouring material of plants, and is vital for photosynthesis (the conversion of the sun light energy is to help, food for the plant).
Deficiencies occur mainly on sandy acid soils in high rainfall areas, especially if used for intensive horticulture activities or dairy production. Heavy applications of potassium in fertilisers can also produce magnesium deficiency, so banana growers need to watch magnesium levels because bananas are big potassium users.
Magnesium deficiency can be overcome with dolomite (a mixed magnesium-calcium carbonate), magnesite (magnesium oxide) or epsom salts (magnesium sulfate).
Function of Magnesium (Mg) in Plants:
- It is essential for all green plants.
- Stabilizes the ribosome particles in the configuration for protein synthesis.
- Helps in uptake of phosphorus and regulates uptake of other nutrients.
- It’s a component of the chlorophyll molecule
- Enzymes: Act as a cofactor in most enzymes that activate phosphorylation processes as a bridge between pyrophosphate structures of ATP or ADP and the enzyme molecule.
Content and distribution in Plants: Content in leaves increases with age, the highest concentrations being found in older leaves.
Root absorption:
- Available forms: Exists as the Mg2+ cation in the soil solution.
- Movement in soil: Supply to the roots depends on root interception, mass flow, and diffusion, with mass flow being the primary delivery mechanism.
Nutrient Deficiency in Plants – symptoms:
- Mg is a mobile element in the plants so it creates deficiency, yellowing of leaves or interveinal chlorosis, which begins on the older leaves.
- On severe condition: Development of necrosis symptoms will appear on the younger leaves when the deficiency is very severe.
- Mg deficiency can be induced by high concentrations of either the NH4+, K+, or Ca2+ cations in the rooting medium, as the Mg2+ cation is the poorest competitor among these three cations
Fertilizer sources:
For acid soils, and as for Ca, it is generally assumed that maintaining the soil pH within the optimum range (5.8 to 7.5) by frequent liming using dolomitic (Mg-bearing) limestone or other high-content-Mg liming materials, will provide sufficient Mg to meet crop requirements.
Sulfur (S)
Established Year: 1866
Researcher(s): Bimer and Lucanus
Existence:
Plant process: Sulfur is a constituent of amino acids in plant proteins and is involved in energy-producing processes in plants. It is responsible for many flavor and odour compounds in plants such as the aroma of onions and cabbage.
Sulfur leaches easily, soils containing high in organic matter.
Source: Superphosphate, gypsum, elemental sulfur and sulfate of ammonia are the main fertiliser sources.
Function of Sulfur (S) in plants:
- It stimulates root growth, seed formation and nodule formation.
- Involved in protein synthesis.
- Amino acid: It’s a part of the amino acids cystine and thiamine.
- Is present in peptide glutathione, coenzyme A, and vitamin B1, and in glucosides such as mustard oil and thiols, which contribute the characteristic odor and taste to plants in the Cruciferae and Liliaceae families.
- Reduces the incidence of disease in many plants
- Interaction with other elements:
- The N-to-S ratio may be as important as total S alone or the ratio of sulfate-sulfur (SO4-S) to total S as indicators of S sufficiency.
- Cruciferae accumulate three times as much S as P.
- Leguminosae accumulate equal amounts of S and P.
- Cereals accumulate one-third less S than P.
Root absorption:
- Available forms:
- Soil organic matter: As decomposition rate of soil organic matter Over 90% of available S exists, in which approximate 10:1 Nitrogen:Sulfur (N:S) ratio available.
- In soil solution: The sulfate (SO42–) anion is the primary available form found it.
- In general, most of the available SO4 is found in the subsoil as the anion and can be easily leached from the surface horizon.
- Soil, the subsoil contains most of the SO4, available as the anion and can be easily leached from the surface horizon.
- Availability may depend on that deposited in rainfall (acid rain) and/or that released from organic matter decomposition.
- At high soil pH (>7.0), S may be precipitated as calcium sulfate (CaSO4), while at lower pH levels (<4.0), the SO42– anion may be adsorbed by Al and Fe oxides.
- Plant availability is influenced by soil water pH.
- 2 Movement in soil:
- Moves in the soil as the SO42– anion by mass flow and within the soil solution by diffusion.
- Low soil-moisture conditions can inhibit S uptake.
- Sulfur precipitation: Sulfate may precipitate as CaSO4 (calcium sulfate) around the roots if mass flow brings SO42– anions at a rate greater than what can be absorbed.
Nutrient Deficiency in Plants – symptoms:
- Whole plant: Sulfur (S) symptoms normally affect the whole plant. Light yellow-green in foliage color initially over the entire plant.
- Plant’s part:
- Roots: Roots are longer than normal and root nodulation in legumes is reduced.
- Stem: stems become woody than normal.
- Grains: delayed maturity occurs in grains.
Interesting Note: Exception in Tobacco, to get proper leaf color, S deficiency is desired in tobacco.
Toxicity: Excess symptoms– Premature senescence of leaves may occur.
Fertilizer sources:
- Atmospheric deposition of S can occur downwind of large cities and industrial plants sufficient to meet S-crop requirements.
- Is becoming an increasingly occurring deficiency in many agricultural areas due to reduced atmospheric deposition and the use of low S-content fertilizers.
Trace Elements
Other important nutrients are Calcium, Magnesium and Sulfur. Plants also need small quantities of Iron, Manganese, Zinc, Copper, Boron and Molybdenum, known as trace elements because only small traces are needed by the plant to strength the stem. The role of these nutrients play in plant growth is complex.
Iron (Fe)
Established Year: 1843
Researcher(s): Gris
Existence:
Iron is a constituent of many compounds that regulate and promote growth and development.
Function of Iron (Fe):
- Essential for formation of chlorophyll and synthesis of proteins and several metabolic reactions
Fertilizer sources:
• Ferrous ammonium phosphate; Fe(NH4)PO4.H2O; 29% Fe
• Ferrous ammonium sulfate; (NH4) 2SO4.FeSO4.6H2O; 14% Fe
• Ferrous sulfate; FeSO4.7H2O; 19% to 21% Fe
• Ferric sulfate; Fe(SO4)3•4H2O; 23% Fe
• Iron chelates:
• NaFeEDTA; 5% to 11% Fe
• NaFeHFDTA; 5% to 9% Fe
• NaFeEDDHA; 6% Fe
• NaFeDTPA; 10% Fe
• Iron polyflavonoids; organically bound Fe; 9% to 10% Fe
Manganese (Mn)
Established Year: 1923
Researcher(s): McHargue
Existance: Soil content – 200 to 400 mg Mn/kg (extreme values: 12 to 10,000 mg Mn/kg). In soil solution as either Mn2+, Mn3+, or Mn4+ cations and as exchangeable Mn.
Photosynthesis process: Manganese helps in it through the chlorophyll.
Toxicity: Manganese found in toxic amounts in very acid soils, but can be deficient in sandy soils. Toxicity is remedied with lime.
Function of Manganese (Mn) in plants:
- It helps in chlorophyll formation.
- Involved in oxidation-reduction processes in the photosynthetic electron transport system.
- Essential in photosystem II for photolysis (acts as a bridge for ATP and enzyme complex phosphokinase and phosphotransferases, and activates IAA oxidases)
Relative sensitivity to manganese by crop species:
- Low Sensitivity: asparagus, blueberry, cotton, rye
- Moderate Sensitivity: alfalfa, barley, broccoli, cabbage, carrot, cauliflower, celery, clover, cucumber, corn, grass, parsnips, peppermint, sorghum, spearmint, sugar beet, tomato, turnip
- High Sensitivity: beans, citrus, lettuce, oats, onion, pea, peach, potato, radish, soybean, spinach, Sudan grass, table beet, wheat
Root absorption:
- 1. Available forms of manganese:
- Existence: In soil solution as either Mn2+, Mn3+, or Mn4+ cations and as exchangeable Mn. Cation Mn2+ is the ionic form taken up by plants.
- Soil pH Availability: Significantly affected by soil pH, decreasing – when the pH increases above 6.2 in some soils; while in other soils, the decrease may not occur until the soil water pH reaches 7.5
- Soil temperatures: Mn can reduce soil temperature significantly to low level.
- Organic matter content: Soil organic matter can reduce Mn availability, decreasing its availability with an increase in organic matter content.
- 2 Movement in soil (Mn):
- In plant’s soil, root absorption supplied by diffusion and root interception.
- Low soil temperature and moisture stress will reduce Mn uptake.
- Some plants may release root exudates that reduce Mn4+ to Mn2+, complex it, thereby increasing Mn availability to the plant.
Deficiency symptoms:
Leaves-
- Dicot Leaves: reduced or stunted growth with visual interveinal chlorosis on the younger leaves is symptomatic of Mn deficiency.
- Cereals develop gray spots on their lower leaves (gray speck),
- legumes develop necrotic areas on their cotyledons (marsh spot).
Toxicity: Excess symptoms:
- Excess of Mn creates brown spots: In older leaves excess of Mn creates brown spots surrounded by a chlorotic zone or circle.
- Mn content in the tissue: If high, creates-
- Apple: Black specks on stone fruits and similar black specks on young bark referred to as measles.
Fertilizer sources:
• Manganese sulfate; MnSO4•4H2O; 26% to 28% Mn
• Manganese oxide; MnO; 41% to 68% Mn
• Manganese chelate; MnEDTA; 5% to 12% M
Copper (Cu)
Established Year: 1925
Researcher(s): Mc Hargue
Existance: Exists in the soil primarily in complexed forms as low-molecular-weight organic compounds such as humic and fulvic acids. 5 to 100 mg Cu/kg (extreme values: 0.1 to 1,300 mg Cu/kg)
Copper is an essential constituent of enzymes in plants, although it can be deficient in red soils. Overuse of another trace element- molybdenum, contains fertilizers directly to plant soil or soil area, can cause Copper deficiency in animals.
Toxicity : Toxicity can be a problem for horticulture planting who regularly use Bordeaux mixture or copper oxychloride sprays to control diseases on horticultural crops.
Function of Copper (Cu) in plants:
- It regulates respiratory activities in plants.
- Constituent of the chloroplast protein plastocyanin.
- In photosynthesis process (photosystems I and II), Cu serves as a part of the electron transport system.
- Cu participates in protein and carbohydrate metabolism and nitrogen (N2) fixation too.
- In enzymes: Cu is part of it that reduces both the atoms of molecular oxygen (O2) (cytochrome oxidase, ascorbic acid oxidase, and polyphenol oxidase).
- In Fatty acids: Cu is also involved in the desaturation and hydroxylation of fatty acids.
Root Absorption:
- Available forms for root absorption:
- Soil solution: Cupric ion (Cu2+) is present in very small quantity.
- Uptake availability: lower than, most other micronutrients.
- Movement in soil:
- Concentration: very low (<0.2 mg/kg) in soil (soil solution)
- by diffusion (soil solution)
- Most soils are able to maintain sufficient Cu2+ ions in soil solution even with increasing soil pH to meet crop requirements, if increasing organic matter content, Cu availability can be significantly reduced.
Nutrient Deficiency Symptoms in plants:
- Reduced or stunted growth with distortion of young leaves
- Necrosis of the apical meristem.
- In trees, deficiency may cause white tip or bleaching of younger leaves.
Toxicity: Excess symptoms
- Leaf: Can induce Fe deficiency and leaf chlorosis.
- Root: Suppressed root growth, with inhibited elongation and lateral root formation at relatively low Cu levels in the soil solution.
- Copper is about 5-10 times more toxic to roots than Al
Fertilizer sources:
• Copper sulfate (monohydrate); CuSO4•H2O; 35% Cu
• Copper sulfate (pentahydrate); CuSO4•5H2O; 25% Cu
• Cupric oxide; CuO; 75% Cu
• Cuprous oxide; Cu2O; 89% Cu
• Cupric ammonium phosphate; Cu(N4)PO4•H2O; 32% Cu
• Basic copper sulfates; CuSO4•3Cu(OH)2 (general formula); 13% to 53% Cu
• Cupric chloride; CuCl2; 17% Cu
• Copper chelates; Na2CuEDTA, NaCuHEDTA, organically bound Cu; 5% to 7% Cu
Zinc (Zn)
Established Year: 1926
Researcher(s): Sommer and Lipman
Existence: 10 to 500 mg Zn/kg (extreme values: 4 to 10,000 mg Zn/kg)
Stimulates growth: Zinc helps in the production of a plant hormone responsible for stem elongation and leaf expansion. It is readily available in acid soils, but combines easily with iron in red soils. This is easily cured with the addition of zinc sulfate or crushed zinc minerals. Fruit trees can be sprayed with zinc.
Function of Zinc (Zn) in plants:
- It helps information of growth hormones and chlorophyll.
- High Zn can induce an Fe deficiency, particularly those sensitive to Fe.
- Involved in the same enzymatic functions as Mn and Mg with only carbonic anhydrase activated by Zn.
- High P can interfere with Zn metabolism as well as affect the uptake of Zn through the root as studied suggested.
Root absorption:
1. Available forms:
- Exists in the soil solution as the Zn2+ cation, as exchangeable Zn, and as organically complexed Zn. Availability is affected by soil pH, decreasing with increasing pH
2. Movement in soil:
- In roots by mass flow and diffusion, with diffusion being the primary delivery mechanism.
- Cu2+ and other cations, such as NH4+, will inhibit root Zn uptake.
- P appears to inhibit translocation rather than directly inhibiting uptake.
- Efficiency of Zn uptake seems to be enhanced by a reduction in pH of the rhizosphere.
Nutrient Deficiency Symptoms in plants:
- Appears as a chlorosis in the interveinal areas of new leaves.
- Severe deficiency: Leaf and plant growth become stunted (rosette), and leaves die and fall off the plant.
- At branch terminals of fruit and Nut trees, rosetting occurs with considerable die-back of the branches.
Toxicity: Excess symptoms-
- Plants particularly sensitive to Fe will become chlorotic when Zn levels are abnormally high (>100 ppm).
- There are some plant species that can tolerate relatively high Zn contents (100 to 250 ppm) without any significant effect on plant growth and yield.
Fertilizer sources:
• Zinc sulfate; ZnSO4•7H2O; 35% Zn
• Zinc oxide; ZnO; 78% to 80% Zn
• Zinc chelates:
• Na2ZnEDTA; 14% Zn
• NaZnTA; 13% Zn
• NaZnHEDTA; 9% Zn
• Zinc polyflavonoids; organically bound Zn; 10% Zn
Boron (B)
Established Year: 1928
Researcher(s): Sommer and Lipman
Existence: Usual soil content- 5 to 100 mg B/kg.
Boron helps with the formation of cell walls in rapidly growing tissue. Deficiency reduces the uptake of calcium and inhibits the plant’s ability to use it. It is chronically deficient in North Coast soils used for horticulture but this is easily remedied with borax applied to the soil.
Function of Boron (B) in plants:
- Helps in uptake of calcium and its efficient use by plants.
- Helps in absorption of nitrogen and is necessary in cell division.
- The synthesis of one of the base for RNA (uracil) formation.
- In cellular activities (e.g., division, differentiation, maturation, respiration,
growth, etc.). - Has long been associated with pollen germination and growth, improving the stability of pollen tubes.
- Relatively immobile in plants.
- Transported primarily in the xylem.
Boron Distribution in plants:
- Boron (B) as a content required by plants based on plant species, may be categorized in three groups based on plant species- Leaf content of monocots (1 to 6 ppm), dicots (20 to 70 ppm), and dicots with latex systems (80 to 100 ppm).
- High Ca content in the plant creates a high B requirement.
- High K plant content accentuates the negative effect of low B tissue levels.
- Exists in the plant as the borate anion (BO33–).
Available forms (B) in Soil
- Exists in the soil solution as the borate anion (BO33–)
- B content in soil, exists in organic residues – plant and microorganism residues, released by residue decomposition
- Primary loss of Boron from soils is by leaching ( Leaching: a technique of removing excess Boron from the surface soil and rooting zone )
Movement of content in soil: By mass flow and diffusion
- Relative tolerance of some crops to boron:
- High: Sugar beet, Garden beet, Alfalfa, Gladiolus, Onion, Turnip, Cabbage, Lettuce, Carrot
- Medium: Sunflower Cotton Radish Field peas Barley Wheat Corn Milo Oats Pumpkin Sweet potato
- Low: Peanut Black walnut Navy bean Pear Apple Peach
Nutrient deficiency in plants – symptoms:
- Abnormal growth of growing points (meristematic tissue) with apical growing points eventually becoming stunted, and then die.
- Auxins accumulate at growing points with leaves and stems becoming brittle
Fertilizer sources:
- Fertilizer borate 48; Na2B4O7•10H2O; 14% to 15% B
- Fertilizer borate granular; Na2B4O7•10H2O; 14% B
- Foliarel; Na2B8O13•4H2O; 21% B
- Solubor; Na2B4O7•4H2O + Na2B10O16•10H2O; 20% B
- Borax; Na2B4O7•10H2O; 11% B
Molybdenum (Mo)
Established Year: 1959
Researcher(s): Arnon and Stout
Existence: 0.5 to 5 mg Mo/kg (extreme values: 0.1 to 80 mg Mo/kg)
Molybdenum helps bacteria and soil organisms convert nitrogen in the air to soluble nitrogen compounds in the soil, so is particularly needed by legumes. It is also essential in the formation of proteins from soluble nitrogen compounds.
Function of Molybdenum in plants:
- It is essential for nitrogen fixing organisms both symbiotic and non-symbiotic.
- Is a component of two major enzyme systems, nitrogenase [involved in the conversion of nitrate (NO3)] and nitrate
reductase [conversion of ammonium (NH4)]. - Availability of nitrogen (N) if the primary form (NH4), then the requirement for Mo is reduced greatly.
Content and distribution:
- Leaf content of Mo is usually less than 1 ppm in the dry matter, due in part to the very low level of the molybdate anion (MnO42–) in the soil solution.
- Normal Mo plant content ranges from 0.34 to 1.5 ppm
- Can be taken up in higher amounts without resulting in toxic effects to the plant.
- High Mo content (>10 ppm Mo) forage can pose a serious health hazard to cattle, particularly dairy cows, which have a sensitive Cu-to-Mo balance requirement.
Root absorption:
Available forms:
- Primary soluble soil form is the molybdate anion (MoO42–)
Movement in soil:
- Mo to roots: By mass flow and diffusion, if Mo level is high in soil mass flow supplies most.
- Both P and Mg will enhance Mo uptake, while SO4 will reduce the uptake of Mo.
- Soil: Mo is strongly absorbed by Fe and Al oxides, the formation of which is pH dependent.
- If NO3 is the primary N source, Mo uptake is higher than if NH4 is equal to or greater than NO3 as the source of N.
Nutrient deficiency in plants – symptoms:
- N deficiency symptoms: lack of dark green foliage color.
- Leaf: Older and middle leaves become chlorotic first, and in some instances, leaf margins are rolled and growth and flower formation is restricted.
- Cruciferae and pulse crops have high Mo requirements
- In cauliflower, the middle lamella of the cell wall is not formed completely when Mo is deficient, with only the leaf rib formed, thereby giving a whip-tail (Mo deficiency) appearance in severe cases
Toxicity: Excess symptoms–
- High plant Mo does not normally affect the plant, but can pose a problem for ruminant animals, particularly dairy cows, that consume plants containing 5 ppm or more Mo.
Fertilizer sources:
• Ammonium molybdate; (NH4)6Mo7O24•2H2O; 54% Mo
• Sodium molybdate; Na2MoO4•2H2O; 39% to 41% Mo
• Molybdenum trioxide; MnO2; 66% Mo
Nickel (Ni)
Established Year: 1987
Researcher(s): Brown, Welsh and Cary
Existence: Exists in most soils at sufficient levels to meet most crop requirements.
Function of Nickel (Ni) in plants
- Component of plant urease.
- Benefits growth of N-fixing plant species.
- Barley (Hordeum vulgare) major cereal grain seeds will not germinate when deficient in Nickel
- Toxicity occurs in plant leaf: Ni content exceeds 10 ppm in plant leaf for Ni-sensitive plants and up to 50 ppm for Ni-tolerant plants.
- Movement and Root absorption method: If Ni content available, absorbtion by roots in form of Ni2+ cation.
- Diffusion is the primary delivery mechanism in plant roots and readily redistributed in the whole plant.
- Uptake can be partially inhibited by high levels of Cu2+ and Zn2+ cations in the soil solution.
Nutrient deficiency in plants – symptoms:
- Significant reduction in shoot growth for Barley (Hordeum vulgare) major cereal grain, oat (Avena sativa), and wheat (T. aestivum) plants.
- Reduced Barley (Hordeum vulgare) grain germination.
- Reduced and delayed leaf expansion.
Fertilizer sources:
- Exists in most soils at sufficient levels to meet most crop requirements; therefore Ni is not generally applied as or with fertilizer.
- Availability is significantly reduced on high pH and lime content soils where soil fixation is likely to occur.
- Major source of Ni is sewage sludge
Reference(s): NCERT | Plant Nutrition and Soil Fertility Manual by J. Benton Jones, Jr.
FAQ about Essential Plant Nutrients
How many Nutrients are essential for plants?
There are 17 Essential Elements that act as essential plant nutrients for growth are: Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), Phosphorus (P), Potassium (K), Sulfur (S), Calcium (Ca), Magnesium (Mg), Boron (B), Chlorine (Cl), Copper (Cu), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), and Zinc (Zn).
Explain the nutrients of the plant?
Plants receive their nutrients mainly from soil, based on their quantitative requirements of the Plants, there are 17 Essential plant nutrients, that can be divided into two broad categories as micro and macro nutrients in plants
Micronutrients: Micronutrients for plant include Boron (B), Chlorine (Cl), Copper (Cu), Iron (Fe), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), and Zinc (Zn).
Macronutrients: The macronutrients include Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), Phosphorus (P), Potassium (K), Sulfur (S), Calcium (Ca), Magnesium (Mg).
Where do plants receive their nutrients mainly from?
Plants receive their nutrients mainly from soil and this soil act as a living source for plants to fulfil the nutrients necessity, needed by plants, for their normal growth, development and reproduction.
More Topics: 17 Nutrients and Role of Manures and Fertilizers